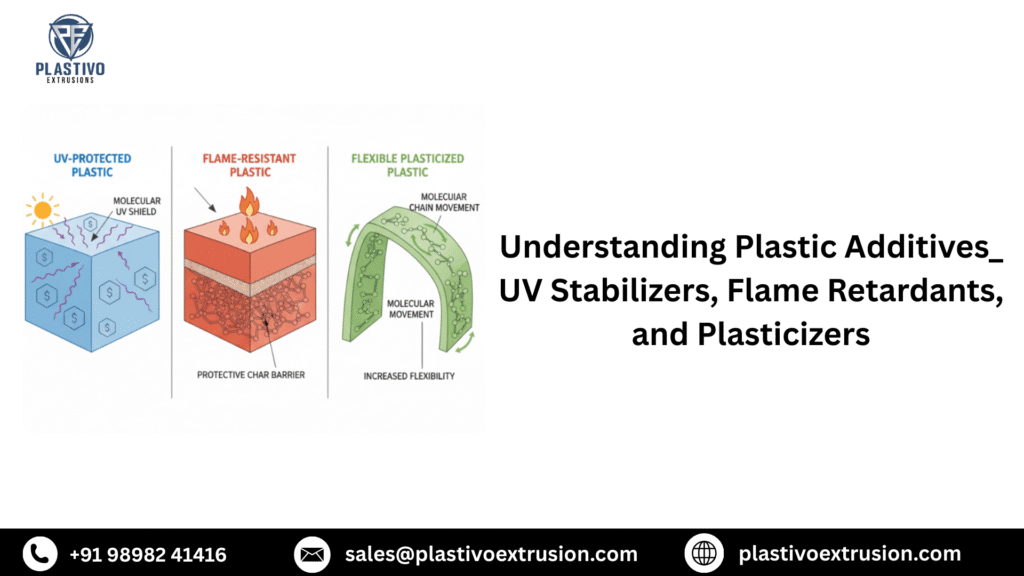
Understanding Plastic Additives_ UV Stabilizers, Flame Retardants, and Plasticizers
Ahmedabad, Gujarat GST No.-24AAVHA8297E1ZU +91 98982 41416 Linkedin-in Instagram Whatsapp Home About us ☰ Our Product Auxiliary Equipment Extruders Pipe Making Machine Plastic Recycling Machine Screw Barrel Blogs Contact us Home About us ☰ Our Product Auxiliary Equipment Extruders Pipe Making Machine Plastic Recycling Machine Screw Barrel Blogs Contact us Request Appointment Understanding Plastic Additives_ UV Stabilizers, Flame Retardants, and Plasticizers Plastic polymers alone rarely meet all the performance requirements of modern applications. That’s where additives come in. These specialized chemicals transform base resins into high-performance materials that can withstand sunlight, resist fire, maintain flexibility, and meet countless other demanding specifications. Understanding how UV stabilizers, flame retardants, and plasticizers work—and how to select the right ones—can mean the difference between product success and costly field failures. Let’s explore these three critical additive categories that shape the plastics industry. UV Stabilizers: Protecting Plastics from Sunlight Degradation Why UV Protection Matters Ultraviolet radiation from sunlight is one of the most destructive forces plastics face. UV exposure breaks down polymer chains through a process called photodegradation, causing materials to become brittle, discolored, chalky, and mechanically weakened. For outdoor applications, UV protection isn’t optional—it’s essential for product longevity. How UV Degradation Occurs When UV light strikes plastic, it provides energy that breaks chemical bonds in the polymer backbone. This creates free radicals that trigger chain reactions, progressively destroying the material. The result? Fading colors, surface cracking, loss of impact strength, and eventual complete failure. Different polymers have varying susceptibility to UV damage. Polypropylene, polystyrene, and ABS are particularly vulnerable, while materials like polycarbonate and acrylic have better inherent UV resistance. Types of UV Stabilizers UV Absorbers (UVAs) UV absorbers work like sunscreen for plastics. They absorb harmful UV radiation and dissipate it as harmless heat before it can damage the polymer chains. Common types include: Benzotriazoles – Excellent for thin sections, widely used in clear and light-colored plastics Benzophenones – Cost-effective, good for thick sections but can yellow over time Triazines – Superior performance in polycarbonate and polyester applications Typical loading: 0.1-2.0% by weight Best applications: Clear or translucent products, automotive glazing, outdoor furniture, agricultural films Hindered Amine Light Stabilizers (HALS) HALS don’t absorb UV light. Instead, they act as radical scavengers, neutralizing the free radicals created by UV exposure before they can cause chain scission. They’re remarkably effective at very low concentrations. Key advantages: Long-lasting protection (regenerate during use) Don’t discolor the plastic Effective at very low loading levels Synergistic when combined with UV absorbers Typical loading: 0.05-0.5% by weight Best applications: Polyolefins (PP, PE), automotive parts, outdoor equipment, geotextiles Combination Approach Many high-performance formulations use both UV absorbers and HALS together. The UV absorber reduces the amount of harmful radiation reaching the polymer, while HALS neutralize any free radicals that do form. This dual-action approach provides superior long-term protection. Selection Considerations When choosing UV stabilizers, consider: Application environment – Desert sun requires more protection than partial shade Expected lifetime – 5-year warranty vs. 20-year warranty needs different additive levels Polymer type – Different stabilizers work better with specific resins Color requirements – Some stabilizers can cause yellowing in clear or white plastics Regulatory compliance – Food contact, toys, and medical applications have restrictions Cost constraints – Balance protection needs with budget realities Testing and Validation Accelerated weathering tests using QUV or Xenon arc chambers can predict outdoor performance. However, natural weathering at test sites in Arizona or Florida provides the most reliable long-term data. Plan for 12-24 months of outdoor exposure testing for critical applications. Flame Retardants: Engineering Fire Safety into Plastics The Fire Safety Challenge Plastics are organic materials that can burn. As they’ve replaced traditional materials like metal and wood in applications from electronics to construction, fire safety has become paramount. Flame retardants reduce ignition risk, slow flame spread, and can even cause self-extinguishing behavior. How Flame Retardants Work Flame retardants employ several mechanisms to inhibit combustion: Gas Phase Action – Release gases that dilute flammable volatiles and interrupt combustion chemistry Condensed Phase Action – Form protective char layers that insulate the underlying plastic from heat Heat Sink Effect – Endothermic decomposition absorbs heat, cooling the material below ignition temperature Physical Barrier – Create barriers that prevent oxygen from reaching the combustion zone Major Types of Flame Retardants Halogenated Flame Retardants These bromine or chlorine-based additives are highly effective and widely used, particularly in electronics. Common types: Brominated compounds (TBBPA, DecaBDE replacements, brominated polystyrene) Chlorinated compounds (chlorinated paraffins, though facing restrictions) Advantages: Highly effective at low loading levels (5-15%) Minimal impact on mechanical properties Cost-effective Well-established performance data Challenges: Environmental and health concerns have led to restrictions on some types Can produce corrosive gases during combustion Increasing regulatory scrutiny globally Best applications: Electronics enclosures, circuit boards, wire and cable insulation, business equipment Phosphorus-Based Flame Retardants Phosphorus compounds work primarily by promoting char formation and releasing gases that dilute combustion. Common types: Red phosphorus – Very effective but moisture-sensitive Organophosphates (TPP, RDP, BDP) Phosphonates and phosphinates (e.g., aluminum diethylphosphinate) Advantages: Lower environmental concerns than halogenated types Good smoke suppression properties Effective in engineering resins Challenges: Can affect polymer processing Some types have hydrolytic stability issues May impact mechanical properties more than halogenated versions Best applications: Engineering plastics, polyurethane foams, thermoset resins, textiles Mineral Flame Retardants These inorganic additives work through endothermic decomposition and dilution mechanisms. Common types: Aluminum trihydrate (ATH) – Releases water when heated Magnesium hydroxide (MDH) – Higher decomposition temperature than ATH Antimony trioxide – Used synergistically with halogenated FR Advantages: Low toxicity and smoke generation Environmentally benign Cost-effective Good smoke suppression Challenges: High loading levels required (40-65%) Significant impact on mechanical properties Can affect processing and surface finish Best applications: Wire and cable compounds, polyolefin formulations, thermoset composites Intumescent Flame Retardants These sophisticated systems expand when heated, creating an insulating foam barrier. Components: Acid source (ammonium polyphosphate) Carbonific (pentaerythritol) Blowing agent (melamine) Advantages: Excellent flame barrier properties Low smoke and toxicity Effective in coatings and certain thermoplastics Challenges: More expensive than conventional FR Can affect surface appearance Sensitive to processing conditions Best applications: Intumescent coatings, polypropylene, polyamides, specialized applications Regulatory Landscape The flame retardant landscape is constantly evolving due to regulatory changes:
Understanding Plastic Additives_ UV Stabilizers, Flame Retardants, and Plasticizers Read More »